What’s cell mechanics?
Cell mechanics is a field of study that focuses on the mechanical properties and behavior of cells, which are the basic building blocks of life. This field combines principles from physics, engineering, and biology to understand how cells respond to mechanical forces, such as stretching, compression, or shear stress. Cell mechanics has a wide range of applications, including tissue engineering, regenerative medicine, and cancer research.
One of the key areas of research in cell mechanics is the study of cell deformation and shape change. Researchers use techniques such as atomic force microscopy, optical tweezers, and micropipette aspiration to apply controlled mechanical forces to cells and observe how they respond. By studying how cells deform and change shape, researchers can gain insights into the mechanical properties of cells and the underlying biological processes that govern their behavior.
Cell mechanics is also important in the development of tissue engineering and regenerative medicine strategies. Researchers use biomechanical principles to design scaffolds and other structures that can support the growth and function of cells, tissues, and organs. By understanding the mechanical properties of cells and the extracellular matrix, researchers can design scaffolds that mimic the natural mechanical environment of the body, promoting the growth and function of cells and tissues.
In addition to tissue engineering and regenerative medicine, cell mechanics has implications for cancer research. Researchers have found that cancer cells exhibit different mechanical properties than normal cells, such as increased stiffness and decreased deformability. By studying these differences, researchers can develop new diagnostic and therapeutic strategies for cancer, such as mechanical probes that can detect cancer cells in tissue samples or treatments that target the mechanical properties of cancer cells.
Overall, cell mechanics is a diverse and rapidly evolving field that has many practical applications in areas ranging from tissue engineering and regenerative medicine to cancer research and diagnostics. By studying the mechanical properties of cells, researchers can gain new insights into the underlying biological processes that govern cell behavior and develop new technologies and strategies for improving human health and wellbeing.
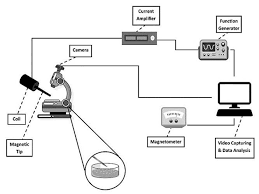
Magnetic Tweezer Cytometery
Magnetic tweezer cytometry is a cutting-edge technique in the field of biophysics and cell biology that allows scientists to manipulate and study biological cells at the microscale level using magnetic forces. This technique has gained prominence due to its non-invasive nature and its ability to provide valuable insights into cellular mechanics and behavior.
At its core, magnetic tweezer cytometry involves the use of magnetic beads that are functionalized and attached to the surface of the cells under study. These magnetic beads are typically coated with specific molecules that bind to receptors on the cell membrane, ensuring a secure attachment. Once the cells are labeled with these beads, an external magnetic field is applied to exert controlled forces on the beads, consequently affecting the cells’ movement and deformation.
The manipulation and measurements achieved through magnetic tweezer cytometry offer several advantages. One of the most significant benefits is its ability to apply precise mechanical forces to cells, allowing researchers to mimic physiological conditions and observe cellular responses in real-time. This approach has provided insights into various cellular processes such as mechanotransduction, cell adhesion, and cellular response to mechanical stimuli.
Additionally, magnetic tweezer cytometry enables the measurement of cell stiffness, elasticity, and deformability. By monitoring how cells respond to mechanical forces, researchers can deduce vital information about the cytoskeletal structure, cell membrane properties, and the overall mechanical integrity of cells. These insights have implications in fields ranging from cancer research to understanding the behavior of immune cells.
Furthermore, the technique is relatively non-invasive compared to traditional methods, as it doesn’t require direct physical contact with the cell or alterations to its environment. This aspect is particularly advantageous when studying delicate or sensitive cells, where traditional tools might cause unwanted perturbations.
In summary, magnetic tweezer cytometry represents a powerful tool in cellular biophysics. It allows researchers to manipulate and measure cellular responses to mechanical forces in a controlled and non-invasive manner. The insights gained from this technique contribute to our understanding of cellular mechanics and behavior, impacting various fields including cell biology, medicine, and biotechnology. As technology advances, magnetic tweezer cytometry is likely to continue playing a pivotal role in unraveling the intricacies of cellular function.
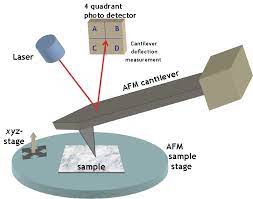
Atomic Force Microscopy
Biologic Atomic Force Microscopy (Biologic AFM) is a cutting-edge scientific technique that has revolutionized the field of biophysics and biochemistry. It merges the principles of atomic force microscopy (AFM) with biological systems to enable high-resolution imaging and analysis of biological structures at the nanoscale.
Atomic Force Microscopy itself is a powerful imaging technique that utilizes a tiny cantilever with a sharp probe tip to scan the surface of a sample. By measuring the interaction forces between the probe tip and the sample, AFM can create detailed topographical maps of surfaces with exceptional resolution. This technique has been instrumental in various scientific fields, particularly in studying materials and nanoscale structures.
Biologic AFM takes this a step further by focusing on biological samples, such as cells, proteins, and DNA molecules. It allows researchers to visualize and manipulate these biological structures at an unprecedented level of detail. With Biologic AFM, scientists can observe cellular processes, molecular interactions, and even changes in biological structures over time.
One of the key advantages of Biologic AFM is its ability to operate under physiological conditions, meaning that samples can be studied in their native environments without the need for extensive preparation or staining. This is crucial for obtaining accurate insights into the behavior of biological molecules and systems.
In practice, Biologic AFM involves delicately positioning a sharp probe above the biological sample. As the probe scans across the surface, interactions between the probe and the sample generate data that is translated into high-resolution images. Additionally, Biologic AFM can be used for force spectroscopy experiments, where the forces between the probe and the sample are measured as the probe is manipulated, providing information about the mechanical properties of biological structures.
The applications of Biologic AFM are vast and impactful. It has been instrumental in studying the structure and dynamics of proteins, understanding the mechanics of cellular membranes, and investigating the interactions between biomolecules. This knowledge has implications for drug development, disease research, and the design of novel biomaterials.
In conclusion, Biologic AFM represents a groundbreaking approach that bridges the gap between traditional atomic force microscopy and the complex world of biological systems. Its ability to provide high-resolution images and data about biological structures at the nanoscale has opened up new avenues of research and understanding in the fields of biophysics, biochemistry, and beyond. As technology continues to advance, Biologic AFM is likely to contribute even more profoundly to our understanding of the intricate mechanisms of life.